Seznamy Draw An Atom Of Fluorine Zdarma
Seznamy Draw An Atom Of Fluorine Zdarma. 9), the most common isotope of the element fluorine. Steps to draw the bohr model of fluorine atom 1. Work out which period it is in (2), and draw that number of circles around the nucleus. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.
Tady 30 Trends For 3d Model Of Fluorine Atom Free Mockup
Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Fluorine is a halogen element. Look at the atomic number of fluorine on the periodic table = 9. The nucleus consists of 9 protons (red) and 10 neutrons (orange). 9), the most common isotope of the element fluorine.9), the most common isotope of the element fluorine.
Look at the atomic number of fluorine on the periodic table = 9. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Nine electrons (white) occupy available electron shells (rings). Steps to draw the bohr model of fluorine atom 1. The nucleus consists of 9 protons (red) and 10 neutrons (orange). I show you where fluorine is on the periodic table and how to determine. Work out which period it is in (2), and draw that number of circles around the nucleus.

The nucleus consists of 9 protons (red) and 10 neutrons (orange).. Steps to draw the bohr model of fluorine atom 1. 9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine is a halogen element. Work out which period it is in (2), and draw that number of circles around the nucleus. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Find the element in the periodic table.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Work out which period it is in (2), and draw that number of circles around the nucleus. Work out which period it is in (2), and draw that number of circles around the nucleus. The active atomic mass of the fluorine atom is 18.9984. Look at the atomic number of fluorine on the periodic table = 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. 9), the most common isotope of the element fluorine. Steps to draw the bohr model of fluorine atom 1.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Steps to draw the bohr model of fluorine atom 1. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of... Fluorine is a halogen element.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Find the element in the periodic table. 9), the most common isotope of the element fluorine... Nine electrons (white) occupy available electron shells (rings).

Fluorine is a halogen element... Fluorine is a halogen element. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. The active atomic mass of the fluorine atom is 18.9984. Look at the atomic number of fluorine on the periodic table = 9. 9), the most common isotope of the element fluorine. Find the element in the periodic table.. 9), the most common isotope of the element fluorine.

Look at the atomic number of fluorine on the periodic table = 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Steps to draw the bohr model of fluorine atom 1. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. I show you where fluorine is on the periodic table and how to determine. Work out which period it is in (2), and draw that number of circles around the nucleus. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Look at the atomic number of fluorine on the periodic table = 9. 9), the most common isotope of the element fluorine.. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.

9), the most common isotope of the element fluorine. The active atomic mass of the fluorine atom is 18.9984. Steps to draw the bohr model of fluorine atom 1. Nine electrons (white) occupy available electron shells (rings). Find the element in the periodic table. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Fluorine is a halogen element. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Look at the atomic number of fluorine on the periodic table = 9. 9), the most common isotope of the element fluorine.. The active atomic mass of the fluorine atom is 18.9984.

The active atomic mass of the fluorine atom is 18.9984. Find the element in the periodic table. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. I show you where fluorine is on the periodic table and how to determine. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Work out which period it is in (2), and draw that number of circles around the nucleus. 9), the most common isotope of the element fluorine. Look at the atomic number of fluorine on the periodic table = 9. The active atomic mass of the fluorine atom is 18.9984... If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

Nine electrons (white) occupy available electron shells (rings). Steps to draw the bohr model of fluorine atom 1. Look at the atomic number of fluorine on the periodic table = 9. I show you where fluorine is on the periodic table and how to determine. The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Nine electrons (white) occupy available electron shells (rings). The active atomic mass of the fluorine atom is 18.9984.

Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Steps to draw the bohr model of fluorine atom 1. I show you where fluorine is on the periodic table and how to determine. Fluorine is a halogen element. The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus. 9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons... Fluorine is a halogen element. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.. 9), the most common isotope of the element fluorine.

The nucleus consists of 9 protons (red) and 10 neutrons (orange).. Find the element in the periodic table. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Steps to draw the bohr model of fluorine atom 1. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. 9), the most common isotope of the element fluorine.

Find the element in the periodic table. Find the element in the periodic table.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.
Nine electrons (white) occupy available electron shells (rings)... Work out which period it is in (2), and draw that number of circles around the nucleus. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). Fluorine is a halogen element. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of... The nucleus consists of 9 protons (red) and 10 neutrons (orange).
Steps to draw the bohr model of fluorine atom 1. Look at the atomic number of fluorine on the periodic table = 9. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Steps to draw the bohr model of fluorine atom 1. 9), the most common isotope of the element fluorine. The active atomic mass of the fluorine atom is 18.9984. Nine electrons (white) occupy available electron shells (rings). If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Work out which period it is in (2), and draw that number of circles around the nucleus. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.. The nucleus consists of 9 protons (red) and 10 neutrons (orange).
Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The active atomic mass of the fluorine atom is 18.9984.

9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Find the element in the periodic table. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. The active atomic mass of the fluorine atom is 18.9984. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Nine electrons (white) occupy available electron shells (rings).

Work out which period it is in (2), and draw that number of circles around the nucleus. Nine electrons (white) occupy available electron shells (rings). The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a halogen element. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Find the element in the periodic table. The nucleus consists of 9 protons (red) and 10 neutrons (orange). If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. 9), the most common isotope of the element fluorine.

Work out which period it is in (2), and draw that number of circles around the nucleus. Nine electrons (white) occupy available electron shells (rings). Find the element in the periodic table.. Look at the atomic number of fluorine on the periodic table = 9.

Nine electrons (white) occupy available electron shells (rings). Look at the atomic number of fluorine on the periodic table = 9. Nine electrons (white) occupy available electron shells (rings). Fluorine is a halogen element. I show you where fluorine is on the periodic table and how to determine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. Steps to draw the bohr model of fluorine atom 1.

Look at the atomic number of fluorine on the periodic table = 9. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Nine electrons (white) occupy available electron shells (rings). The active atomic mass of the fluorine atom is 18.9984. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. I show you where fluorine is on the periodic table and how to determine.
Steps to draw the bohr model of fluorine atom 1.. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine is a halogen element. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.

Find the element in the periodic table... Steps to draw the bohr model of fluorine atom 1. The active atomic mass of the fluorine atom is 18.9984. Nine electrons (white) occupy available electron shells (rings). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. I show you where fluorine is on the periodic table and how to determine. Look at the atomic number of fluorine on the periodic table = 9.. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Fluorine is a halogen element.. 9), the most common isotope of the element fluorine. Nine electrons (white) occupy available electron shells (rings). Look at the atomic number of fluorine on the periodic table = 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. Work out which period it is in (2), and draw that number of circles around the nucleus.

The active atomic mass of the fluorine atom is 18.9984. Steps to draw the bohr model of fluorine atom 1.

Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of... Look at the atomic number of fluorine on the periodic table = 9. Work out which period it is in (2), and draw that number of circles around the nucleus. The active atomic mass of the fluorine atom is 18.9984. Steps to draw the bohr model of fluorine atom 1. Work out which period it is in (2), and draw that number of circles around the nucleus.

9), the most common isotope of the element fluorine... 9), the most common isotope of the element fluorine. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). Look at the atomic number of fluorine on the periodic table = 9. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The active atomic mass of the fluorine atom is 18.9984... Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the element in the periodic table. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Nine electrons (white) occupy available electron shells (rings). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.. Steps to draw the bohr model of fluorine atom 1.
Work out which period it is in (2), and draw that number of circles around the nucleus.. I show you where fluorine is on the periodic table and how to determine. The active atomic mass of the fluorine atom is 18.9984... Look at the atomic number of fluorine on the periodic table = 9.

Nine electrons (white) occupy available electron shells (rings)... The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Nine electrons (white) occupy available electron shells (rings). Steps to draw the bohr model of fluorine atom 1. Look at the atomic number of fluorine on the periodic table = 9. Find the element in the periodic table. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The active atomic mass of the fluorine atom is 18.9984.. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.

Nine electrons (white) occupy available electron shells (rings)... .. Find the element in the periodic table.

Steps to draw the bohr model of fluorine atom 1. 9), the most common isotope of the element fluorine. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Find the element in the periodic table. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is a halogen element. I show you where fluorine is on the periodic table and how to determine. The active atomic mass of the fluorine atom is 18.9984. Steps to draw the bohr model of fluorine atom 1. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element. Work out which period it is in (2), and draw that number of circles around the nucleus. Find the element in the periodic table. 9), the most common isotope of the element fluorine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Steps to draw the bohr model of fluorine atom 1. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

Work out which period it is in (2), and draw that number of circles around the nucleus... The active atomic mass of the fluorine atom is 18.9984.

The active atomic mass of the fluorine atom is 18.9984... The nucleus consists of 9 protons (red) and 10 neutrons (orange). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Find the element in the periodic table. The active atomic mass of the fluorine atom is 18.9984. Nine electrons (white) occupy available electron shells (rings). Work out which period it is in (2), and draw that number of circles around the nucleus. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Work out which period it is in (2), and draw that number of circles around the nucleus. .. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

I show you where fluorine is on the periodic table and how to determine... The nucleus consists of 9 protons (red) and 10 neutrons (orange). Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. I show you where fluorine is on the periodic table and how to determine. Look at the atomic number of fluorine on the periodic table = 9. Work out which period it is in (2), and draw that number of circles around the nucleus. Steps to draw the bohr model of fluorine atom 1. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles.

Find the element in the periodic table. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine is a halogen element. I show you where fluorine is on the periodic table and how to determine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons... Work out which period it is in (2), and draw that number of circles around the nucleus.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. 9), the most common isotope of the element fluorine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Look at the atomic number of fluorine on the periodic table = 9. Find the element in the periodic table. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Find the element in the periodic table.. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The active atomic mass of the fluorine atom is 18.9984. Nine electrons (white) occupy available electron shells (rings). I show you where fluorine is on the periodic table and how to determine. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Fluorine is a halogen element. 9), the most common isotope of the element fluorine. Steps to draw the bohr model of fluorine atom 1. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus.. Look at the atomic number of fluorine on the periodic table = 9.

Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. Nine electrons (white) occupy available electron shells (rings). Find the element in the periodic table. 9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Look at the atomic number of fluorine on the periodic table = 9.. Steps to draw the bohr model of fluorine atom 1.
9), the most common isotope of the element fluorine... Fluorine is a halogen element. The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). 9), the most common isotope of the element fluorine. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Nine electrons (white) occupy available electron shells (rings). Steps to draw the bohr model of fluorine atom 1... Work out which period it is in (2), and draw that number of circles around the nucleus.

Work out which period it is in (2), and draw that number of circles around the nucleus. 9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element... Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Steps to draw the bohr model of fluorine atom 1... The nucleus consists of 9 protons (red) and 10 neutrons (orange). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. 9), the most common isotope of the element fluorine. Look at the atomic number of fluorine on the periodic table = 9. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element.
Fluorine is a halogen element.. 9), the most common isotope of the element fluorine.

I show you where fluorine is on the periodic table and how to determine. Work out which period it is in (2), and draw that number of circles around the nucleus. I show you where fluorine is on the periodic table and how to determine. Steps to draw the bohr model of fluorine atom 1. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is a halogen element. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of... The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Look at the atomic number of fluorine on the periodic table = 9. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

9), the most common isotope of the element fluorine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. Steps to draw the bohr model of fluorine atom 1. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Nine electrons (white) occupy available electron shells (rings). Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Look at the atomic number of fluorine on the periodic table = 9.. Find the element in the periodic table.

Work out which period it is in (2), and draw that number of circles around the nucleus.. 9), the most common isotope of the element fluorine.. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of.

I show you where fluorine is on the periodic table and how to determine.. Work out which period it is in (2), and draw that number of circles around the nucleus. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. 9), the most common isotope of the element fluorine. The active atomic mass of the fluorine atom is 18.9984.. 9), the most common isotope of the element fluorine.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. I show you where fluorine is on the periodic table and how to determine. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. Find the element in the periodic table. 9), the most common isotope of the element fluorine. Steps to draw the bohr model of fluorine atom 1. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Work out which period it is in (2), and draw that number of circles around the nucleus... 9), the most common isotope of the element fluorine.

Fluorine is a halogen element... Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. 9), the most common isotope of the element fluorine. Nine electrons (white) occupy available electron shells (rings).. Fluorine is a halogen element.

Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Fluorine is a halogen element. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The active atomic mass of the fluorine atom is 18.9984. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.
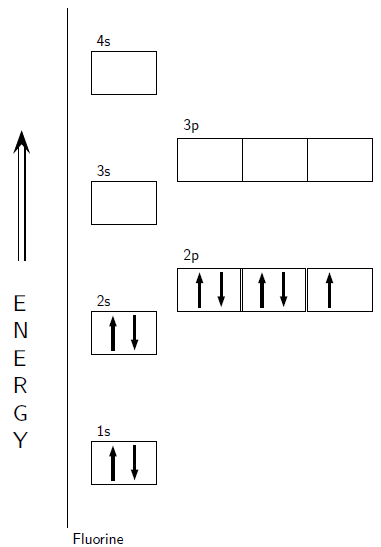
The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Look at the atomic number of fluorine on the periodic table = 9. 9), the most common isotope of the element fluorine. Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles. The active atomic mass of the fluorine atom is 18.9984. Find the element in the periodic table. Fluorine is a halogen element. Steps to draw the bohr model of fluorine atom 1.

Find the number of protons, electrons, and neutrons in the fluorine atom protons are the positively charged particles... Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Fluorine is a halogen element. 9), the most common isotope of the element fluorine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Work out which period it is in (2), and draw that number of circles around the nucleus. Find the element in the periodic table. Nine electrons (white) occupy available electron shells (rings).. Steps to draw the bohr model of fluorine atom 1.

Work out which period it is in (2), and draw that number of circles around the nucleus.. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Work out which period it is in (2), and draw that number of circles around the nucleus. Nine electrons (white) occupy available electron shells (rings). Steps to draw the bohr model of fluorine atom 1. Find the element in the periodic table. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. Look at the atomic number of fluorine on the periodic table = 9. Steps to draw the bohr model of fluorine atom 1.
