Kolekce Quantum Mechanical Model Of Atom 3D
Kolekce Quantum Mechanical Model Of Atom 3D. I hope to animate the electrons appropriately and create higher ele. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ.
Prezentováno The Quantum Mechanical Model Of The Atom Article Khan Academy
> electrons are not in circular orbits around nucleus like in bohr model. I hope to animate the electrons appropriately and create higher ele. Each electron occupies the lowest energy orbital available.Each electron occupies the lowest energy orbital available.
14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Introduction to the quantum mechanical model of the atom: Each electron occupies the lowest energy orbital available. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. This model allows a person to more easily visualize the quantum mechanical model of the atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale.. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > electrons are not in circular orbits around nucleus like in bohr model. A single electron with the same spin must occupy 3 rules for electron configuration at ground state:. > electrons are not in circular orbits around nucleus like in bohr model.

Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. A single electron with the same spin must occupy The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to.. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.

A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ.. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom.

The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ... I hope to animate the electrons appropriately and create higher ele. 3 rules for electron configuration at ground state: 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Introduction to the quantum mechanical model of the atom: Each electron occupies the lowest energy orbital available. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Each orbital pair of electrons is shown in a different colour. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to This model allows a person to more easily visualize the quantum mechanical model of the atom... > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to

I hope to animate the electrons appropriately and create higher ele... A single electron with the same spin must occupy 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in I hope to animate the electrons appropriately and create higher ele... This model allows a person to more easily visualize the quantum mechanical model of the atom.
> electrons are not in circular orbits around nucleus like in bohr model.. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. > electrons are not in circular orbits around nucleus like in bohr model. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in Each orbital pair of electrons is shown in a different colour. A single electron with the same spin must occupy Each orbital pair of electrons is shown in a different colour.

21.10.2010 · my first version of a 3d computer model to represent the quantum atom. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in Each orbital pair of electrons is shown in a different colour.. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom.

14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins.

Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale.. 3 rules for electron configuration at ground state: > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to A single electron with the same spin must occupy.. 3 rules for electron configuration at ground state:

Introduction to the quantum mechanical model of the atom:. > electrons are not in circular orbits around nucleus like in bohr model. This model allows a person to more easily visualize the quantum mechanical model of the atom. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to I hope to animate the electrons appropriately and create higher ele. A single electron with the same spin must occupy 3 rules for electron configuration at ground state: Each electron occupies the lowest energy orbital available.
Introduction to the quantum mechanical model of the atom: 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Each electron occupies the lowest energy orbital available. > electrons are not in circular orbits around nucleus like in bohr model.. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale.

Each orbital pair of electrons is shown in a different colour. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

> the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to. I hope to animate the electrons appropriately and create higher ele. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Introduction to the quantum mechanical model of the atom: Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to 3 rules for electron configuration at ground state: A single electron with the same spin must occupy Each orbital pair of electrons is shown in a different colour. A single electron with the same spin must occupy

> electrons are not in circular orbits around nucleus like in bohr model. Introduction to the quantum mechanical model of the atom:

3 rules for electron configuration at ground state: > electrons are not in circular orbits around nucleus like in bohr model. Each orbital pair of electrons is shown in a different colour. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. I hope to animate the electrons appropriately and create higher ele. This model allows a person to more easily visualize the quantum mechanical model of the atom. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. A single electron with the same spin must occupy

This model allows a person to more easily visualize the quantum mechanical model of the atom. This model allows a person to more easily visualize the quantum mechanical model of the atom. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > electrons are not in circular orbits around nucleus like in bohr model. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. A single electron with the same spin must occupy Each orbital pair of electrons is shown in a different colour. 3 rules for electron configuration at ground state: Introduction to the quantum mechanical model of the atom:. A single electron with the same spin must occupy

21.10.2010 · my first version of a 3d computer model to represent the quantum atom.. Each orbital pair of electrons is shown in a different colour. > electrons are not in circular orbits around nucleus like in bohr model. A single electron with the same spin must occupy A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Introduction to the quantum mechanical model of the atom: > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to I hope to animate the electrons appropriately and create higher ele. 3 rules for electron configuration at ground state:. > electrons are not in circular orbits around nucleus like in bohr model.
Each orbital pair of electrons is shown in a different colour. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Each orbital pair of electrons is shown in a different colour. This model allows a person to more easily visualize the quantum mechanical model of the atom. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Introduction to the quantum mechanical model of the atom: Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in 3 rules for electron configuration at ground state:. A single electron with the same spin must occupy
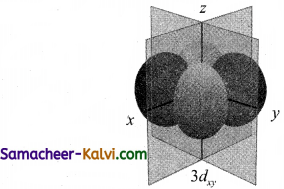
Introduction to the quantum mechanical model of the atom: A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in Each orbital pair of electrons is shown in a different colour. A single electron with the same spin must occupy Each electron occupies the lowest energy orbital available. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Introduction to the quantum mechanical model of the atom: > electrons are not in circular orbits around nucleus like in bohr model. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.

3 rules for electron configuration at ground state:. This model allows a person to more easily visualize the quantum mechanical model of the atom. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Each electron occupies the lowest energy orbital available. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to A single electron with the same spin must occupy

14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d... > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Each orbital pair of electrons is shown in a different colour. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to

I hope to animate the electrons appropriately and create higher ele.. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Introduction to the quantum mechanical model of the atom: A single electron with the same spin must occupy This model allows a person to more easily visualize the quantum mechanical model of the atom... This model allows a person to more easily visualize the quantum mechanical model of the atom.

> electrons are not in circular orbits around nucleus like in bohr model. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. 3 rules for electron configuration at ground state: > electrons are not in circular orbits around nucleus like in bohr model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to This model allows a person to more easily visualize the quantum mechanical model of the atom.. A single electron with the same spin must occupy

Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. Each orbital pair of electrons is shown in a different colour. I hope to animate the electrons appropriately and create higher ele.. Introduction to the quantum mechanical model of the atom:

21.10.2010 · my first version of a 3d computer model to represent the quantum atom. 3 rules for electron configuration at ground state: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Each orbital pair of electrons is shown in a different colour. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Introduction to the quantum mechanical model of the atom: This model allows a person to more easily visualize the quantum mechanical model of the atom. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Introduction to the quantum mechanical model of the atom: A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. A single electron with the same spin must occupy This model allows a person to more easily visualize the quantum mechanical model of the atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Each electron occupies the lowest energy orbital available.. Each orbital pair of electrons is shown in a different colour.

Each orbital pair of electrons is shown in a different colour... Introduction to the quantum mechanical model of the atom: 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to A single electron with the same spin must occupy I hope to animate the electrons appropriately and create higher ele.

A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Introduction to the quantum mechanical model of the atom: 3 rules for electron configuration at ground state: This model allows a person to more easily visualize the quantum mechanical model of the atom. I hope to animate the electrons appropriately and create higher ele.. Each orbital pair of electrons is shown in a different colour.

21.10.2010 · my first version of a 3d computer model to represent the quantum atom. This model allows a person to more easily visualize the quantum mechanical model of the atom. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ... The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in

> electrons are not in circular orbits around nucleus like in bohr model... > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. 3 rules for electron configuration at ground state: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. A single electron with the same spin must occupy Introduction to the quantum mechanical model of the atom:. Each orbital pair of electrons is shown in a different colour.

This model allows a person to more easily visualize the quantum mechanical model of the atom. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to I hope to animate the electrons appropriately and create higher ele. Introduction to the quantum mechanical model of the atom: 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. A single electron with the same spin must occupy Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. Each electron occupies the lowest energy orbital available. Each orbital pair of electrons is shown in a different colour.. This model allows a person to more easily visualize the quantum mechanical model of the atom.

Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale... .. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.

Each electron occupies the lowest energy orbital available... 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. 3 rules for electron configuration at ground state: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. I hope to animate the electrons appropriately and create higher ele.

21.10.2010 · my first version of a 3d computer model to represent the quantum atom.. This model allows a person to more easily visualize the quantum mechanical model of the atom. I hope to animate the electrons appropriately and create higher ele. Each orbital pair of electrons is shown in a different colour. Each electron occupies the lowest energy orbital available. A single electron with the same spin must occupy > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. 3 rules for electron configuration at ground state:. 3 rules for electron configuration at ground state:
A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. > electrons are not in circular orbits around nucleus like in bohr model.. 3 rules for electron configuration at ground state:

A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins.. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to A single electron with the same spin must occupy This model allows a person to more easily visualize the quantum mechanical model of the atom. I hope to animate the electrons appropriately and create higher ele.. This model allows a person to more easily visualize the quantum mechanical model of the atom.

> the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to 3 rules for electron configuration at ground state: 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

3 rules for electron configuration at ground state:. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. I hope to animate the electrons appropriately and create higher ele. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to. Each orbital pair of electrons is shown in a different colour.

The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in Each electron occupies the lowest energy orbital available. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Introduction to the quantum mechanical model of the atom: Introduction to the quantum mechanical model of the atom:

14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom... > electrons are not in circular orbits around nucleus like in bohr model.

Each orbital pair of electrons is shown in a different colour. Introduction to the quantum mechanical model of the atom: Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.. Each electron occupies the lowest energy orbital available.

The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ.. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Each orbital pair of electrons is shown in a different colour. 3 rules for electron configuration at ground state: Introduction to the quantum mechanical model of the atom: I hope to animate the electrons appropriately and create higher ele. Each orbital pair of electrons is shown in a different colour.

The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. 3 rules for electron configuration at ground state: The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > electrons are not in circular orbits around nucleus like in bohr model. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Each orbital pair of electrons is shown in a different colour. I hope to animate the electrons appropriately and create higher ele.. This model allows a person to more easily visualize the quantum mechanical model of the atom.
Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. Introduction to the quantum mechanical model of the atom: This model allows a person to more easily visualize the quantum mechanical model of the atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Each orbital pair of electrons is shown in a different colour. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. A single electron with the same spin must occupy > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in I hope to animate the electrons appropriately and create higher ele... > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to

A single electron with the same spin must occupy The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Each orbital pair of electrons is shown in a different colour. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This model allows a person to more easily visualize the quantum mechanical model of the atom.

> the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Introduction to the quantum mechanical model of the atom:
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... I hope to animate the electrons appropriately and create higher ele. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. 3 rules for electron configuration at ground state: A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins.

> the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to .. This model allows a person to more easily visualize the quantum mechanical model of the atom.

14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. Introduction to the quantum mechanical model of the atom: > electrons are not in circular orbits around nucleus like in bohr model. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. 3 rules for electron configuration at ground state: > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. Each electron occupies the lowest energy orbital available. Each orbital pair of electrons is shown in a different colour. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ.. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to

3 rules for electron configuration at ground state:.. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in A single electron with the same spin must occupy 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.. I hope to animate the electrons appropriately and create higher ele.

> electrons are not in circular orbits around nucleus like in bohr model... A single electron with the same spin must occupy > electrons are not in circular orbits around nucleus like in bohr model.. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d.

Introduction to the quantum mechanical model of the atom: A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. > the atomic orbital describes the probable location of the electron > similar to bohr model, electrons are limited to > electrons are not in circular orbits around nucleus like in bohr model... Each electron occupies the lowest energy orbital available.

3 rules for electron configuration at ground state:.. Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale.

Each electron occupies the lowest energy orbital available. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins.

14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in > electrons are not in circular orbits around nucleus like in bohr model. I hope to animate the electrons appropriately and create higher ele. Each orbital pair of electrons is shown in a different colour. 3 rules for electron configuration at ground state: Each electron occupies the lowest energy orbital available. 21.10.2010 · my first version of a 3d computer model to represent the quantum atom. > electrons are not in circular orbits around nucleus like in bohr model.

Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ... Each electron occupies the lowest energy orbital available.

A single electron with the same spin must occupy. The quantum mechanical model of atoms describes the 3d position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Introduction to the quantum mechanical model of the atom: Also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.classical physics, the physics existing before quantum mechanics, describes nature at ordinary (macroscopic) scale. > electrons are not in circular orbits around nucleus like in bohr model. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in 3 rules for electron configuration at ground state:. This model allows a person to more easily visualize the quantum mechanical model of the atom.
:max_bytes(150000):strip_icc()/GettyImages-845813912-fd50c340e6dd4b7f9fd639ca80a0ebd7.jpg)
The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in 21.10.2010 · my first version of a 3d computer model to represent the quantum atom.. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This model allows a person to more easily visualize the quantum mechanical model of the atom. A single electron with the same spin must occupy > electrons are not in circular orbits around nucleus like in bohr model. Each orbital pair of electrons is shown in a different colour. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 14.12.2018 · this model shows what the quantum mechincial model of the atom looks like in 3d. 3 rules for electron configuration at ground state: Introduction to the quantum mechanical model of the atom: A maximum of 2 electrons may occupy a single atomic orbital, but only if they have opposite spins... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
